Should I get a COVID booster shot?
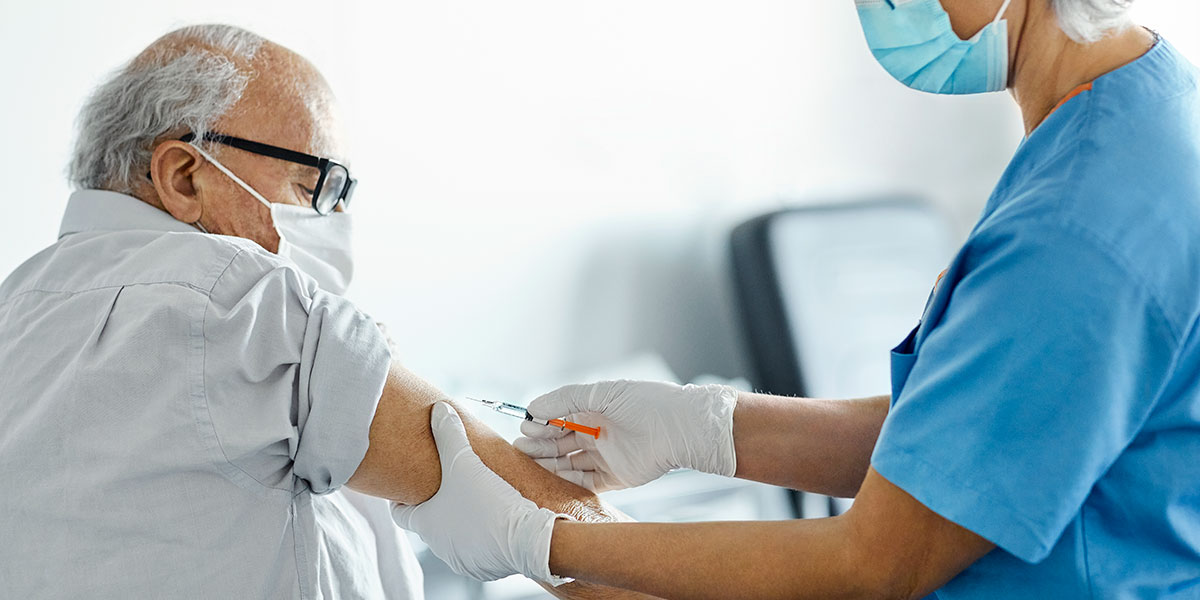
By now you’ve probably heard lots about booster shots for the COVID vaccines. In August, the Food and Drug Administration gave emergency authorization for third shots for people with compromised immune systems – patients receiving chemotherapy and transplant recipients, among others.
At the same time, the White House also announced a plan to begin offering boosters to vaccinated individuals in late September, citing evidence that the vaccines’ effectiveness against infections wanes over time and doesn’t provide as much protection against the Delta variant.
On Sept. 22, the FDA announced a more limited roll out of boosters. Boosters will continue to be available for certain immunocompromised individuals, but the FDA is also recommending that people 65 and older, residents of long-term care facilities and people 50-64 with underlying medical conditions get a booster Pfizer booster shot six months after they’ve completed their initial Pfizer vaccination doses. The government also cleared the way for boosters for people 18 to 49 with underlying medical conditions and those 18 to 64 who are at increased risk for exposure and transmission because of occupation, such as healthcare workers.
For now, the guidance only applies to recipients of Pfizer vaccine, but the CDC said it will address recommendations for Moderna’s and Johnson & Johnson’s vaccines as soon as data is available. Moderna, like Pfizer, has applied for an emergency use authorization for booster shots. J&J has not.
While it may sound like the vaccines — the mRNA vaccines from Pfizer and Moderna and the one-shot Johnson & Johnson adenovirus shot — stop working, this isn’t true. All three vaccines offer excellent protection from severe infection and hospitalization many months after people are fully vaccinated. But studies show protection against less severe infections dissipates over time, and the vaccines are less effective against the Delta variant.
When can I get a COVID booster?
The Centers for Disease Control and Prevention still has to weigh in on timing. Most experts believe the people in the groups identified by the FDA will become eligible for the booster six months after they completed their vaccination. Most people in those groups began receiving their vaccine doses eight months ago.
Which vaccines are authorized?
The only vaccine to receive this emergency use authorization is Pfizer. Moderna has applied for similar consideration. The FDA and CDC have only approved Pfizer boosters for Pfizer recipients.
Pfizer’s mRNA vaccine recently received full authorization for everyone 16 and up. It originally was approved in December under an emergency use authorization; Moderna’s vaccine was also given an EUA in December; and Johnson & Johnson’s vaccine was given an EUA later.
Both Johnson & Johnson and Moderna have released data showing an additional dose of its vaccine increases efficacy against mild to severe COVID. Like Pfizer, Moderna has applied for EUA authorization for booster shots. J&J has not, yet. Studies in Europe have shown that mixing and matching two different types of vaccines – mRNA like Pfizer and Moderna — and AstraZeneca’s adenoviral vaccine produce a strong antibody response and seem to be safe.
Why not everyone else?
An FDA panel that met in mid-September to review evidence supporting additional boosters was uncertain there was enough evidence to authorize booster shots for those 16 to 65 who are healthy. Studies supporting third doses have largely been made of populations 65 and older. The panel also wanted to see more safety data concerning younger adults and a third dose before recommending it broadly.
Finally, members of the advisory panel said the extra dose would not likely slow the current wave of COVID infections. But getting more of the unvaccinated population vaccinated would, and federal efforts should be spent trying to increase the number of vaccinated.
During its review, the CDC determined that certain higher-risk subgroups under age 65 should also get the shot. You can read more about the CDC’s guidance here.
Should I get a COVID Booster?
If you received the second Pfizer vaccine shot more than six months ago and fall into one of the categories listed above, talk to your MDVIP-affiliated physician. He or she will be in the best position to help you make a decision. If you got the Moderna vaccine, there is not good guidance yet on whether you should mix mRNA boosters. Moderna and Pfizer are both mRNA vaccines, but booster studies have only considered people receiving the same mRNA vaccines – not mixing and matching.
There is currently a trial in progress that looks at mixing and matching vaccine booster, but it’s not complete. Moderna applied for a booster exemption after Pfizer, and the government has pledged to review the data and application quickly. J&J has not applied for a booster authorization but has submitted data showing its efficacy.
You’re better off vaccinated
With very rare exception, you’re still better off vaccinated than not. Even though some protection from the vaccines wane over time, they are VERY good at protecting you from severe illness, hospitalization and death. The vast majority of people hospitalized and dying from the Delta variant are unvaccinated.
If you still haven’t been vaccinated, you should get the vaccine. They have been proven safe and effective. If you have concerns about the vaccines, talk to your doctor.





